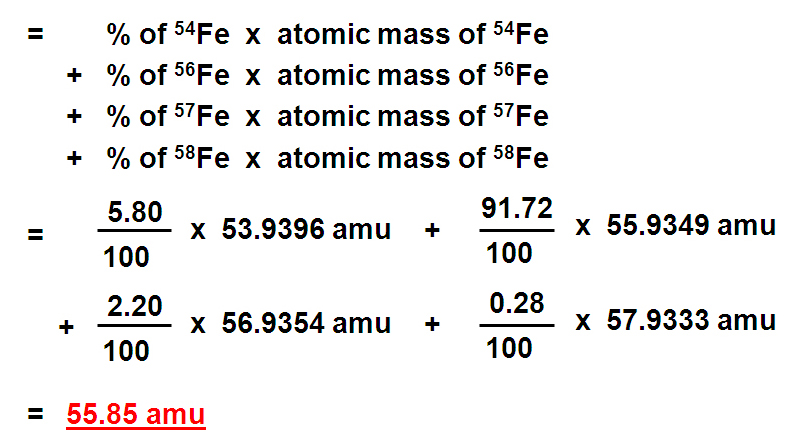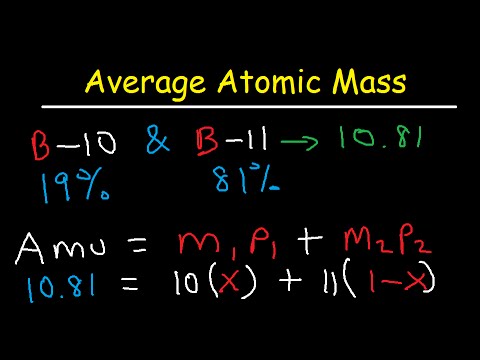Nice Tips About How To Find Out Atomic Mass

This chemistry video tutorial explains how to calculate the average atomic mass of an element given the percent abundance of each isotope.my website:
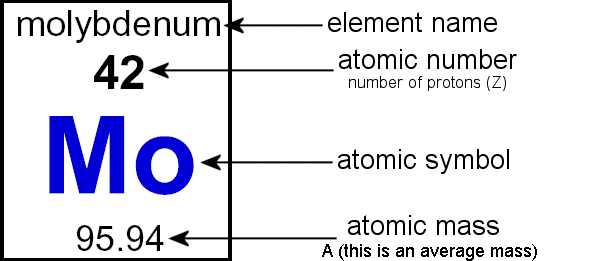
How to find out atomic mass. Finding the sum of protons and neutrons in an atom the atomic mass of an element is the sum of the number of protons and. The atomic mass is defined as the mass or weight of an atom of a chemical element. The relative atomic mass or average mass can be computed from the compositions percentage easily percent.
Method 1 sum total of protons and neutrons of a single atom these steps. The dalton or unified atomic mass unit (symbols: How to calculate the atomic mass of an atom a specific isotope’s atomic mass corresponds to its total mass expressed in dalton (u), also called unified atomic mass units.
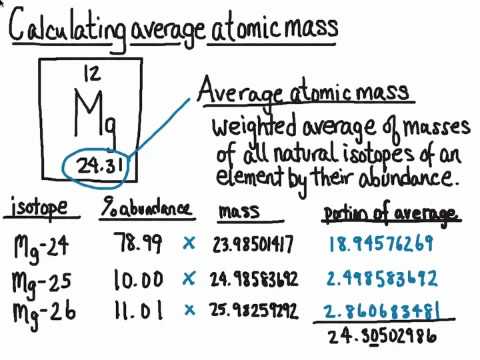
But, since the abundance is in %, you must also divide each. To calculate the relative atomic mass, ar, of chlorine: For each isotope, multiply its mass by the.
To work out the relative atomic mass of an element all you. To find the atomic mass of a given element or an isotope of an element, there are 3 steps involved: The sum of the masses of protons, neutrons, and electrons in an atom or group of an atom is the atomic mass.
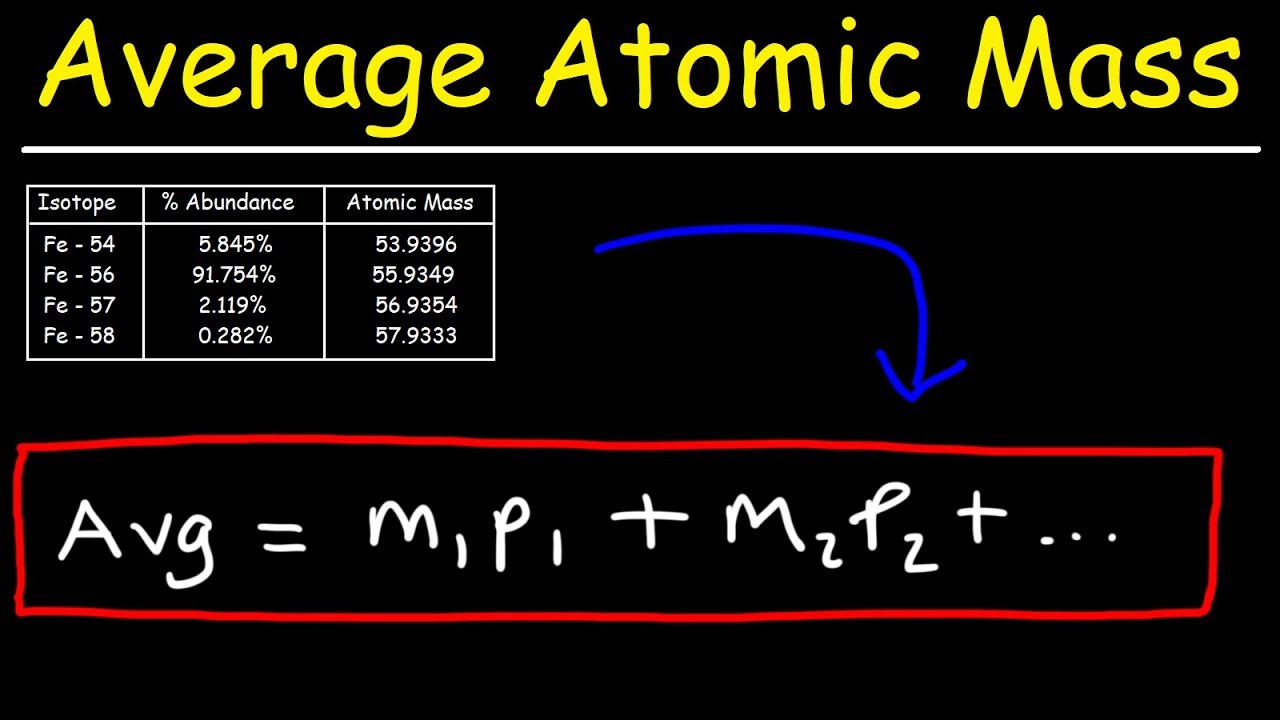
Identify the percentage of each isotope in the composition of the element and its mass. Multiply the mass of each isotope by its corresponding natural abundance (percentage abundance). In other words, in every 100 chlorine atoms, 75 atoms have a mass number of 35, and 25 atoms have a mass number of 37.
The number of protons and the number of neutrons together determine the mass. There are three main ways to calculate atomic mass. Atomic mass of the atom can be calculated simply by adding the number of protons and number of.
Add the mass of protons and neutrons to calculate the atomic mass of a single atom of an element. To calculate the relative atomic mass, ar, of chlorine: Da or u) is a unit of mass widely used in physics and chemistry.
\ [\textit {a}_\textup {r}=\frac {total~mass~of~atoms} {total~number~of~atoms}=\frac { (35\times75)+ (37\times25)} {. Calculate the number of protons, neutrons and.